Group A
1. MCQ type question ( All questions are compulsory ) :
( https://forms.gle/ePqysL8Ab8CzDk3S6 )
Each of the following questions has four alternatives . Write the correct one .
1.1 Which one of the following does not deplete ozone layer ?
a) NO b) N₂O c) CO₂ d) CFC
1.2 Which of the following is equal to 290K ?
a) 30ºC b) 17ºC c) 0ºC d) 27ºC
1.3 The molecular weight of methene is 16 , which of the following is its vapour density ?
a) 22.4 b) 8 c) 16 d) 32
1.4 Which of the following has the highest thermal conductivity in cal.cm⁻¹s⁻¹K⁻¹ unit ) ?
a) Copper b) Gold c) Iron d) Diamond
1.5 For which angle of incidence deviation is minimum during refraction of light from lighter to denser medium ?
a) 60º b) 0º c) 90º d) 45º
1.6 Which pair of the following is the two terminal colours in pure spectrum of while light ?
a) Red and violet b) Red and green c) Violet and orange d) Blue and indigo
1.7 Which one of the following is the unit of electric charge ?
a) volt b) coulomb c) ohm d) watt
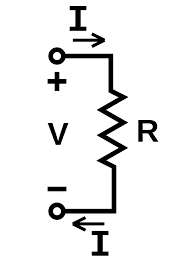
1.8 What will be the equivalent resistance where two resistances of 3 ohm and 6 ohm are connected in parallel combination ?
a) 3 ohm b) 4 ohm c) 2 ohm d) 1 ohm
1.9 Which of the following is the correct order of decreasing power of ionising of gas ?
a) α >β >४ b) ४ > β > ∝ c) α >४ > β d) ४ > α > β
1.10 Which of the following is not a transition element ?
a) Fe b) Co c) Ca d) Cr
1.11 Which one of the following is an ionic compound ?
a) HCl b) CH₄ c) MgCl₂ d) NH₃
1.12 Which of the following is a weak electrolyte ?
a) CuSO₄ b) KOH c) H₂SO₄ d) CH₃COOH
1.13 Which of the following gases can be prepared by Kipp's apparatus ?
a) N₂ b) H₂S c) HCl d) NH₃
1.14 Hematite is the one ore of which of the following metals ?
a) Copper b) Aluminium c) Iron d) Zinc
1.15 Which of the following functional groups is present in ethyl alcohol ?
a) 一CHO b) >C=O c) 一COOH d) 一OH
Group - B
2. Answer the following questions(Alternative are to be noted ) 1 x 21 =21
2.1 In which layer of atmosphere do storm and rains occur ?
Or
What are the bacteria called which decompose biomass to methane in biogas plant ?
2.2 Fill up the blank : In stratosphere temperature .............................with increase in altitude .
2.3 What is the temperature in ºC at which volume of a gas will be zero at constant pressure according to Charles' law ?
2.4 A gas at fixed temperature is kept in a closed vessel . Some more amount of the same gas is added to the vessel without altering the temperature .What will be the change in pressure ?
2.5 What is the SI unit of coefficient of linear expansion of a solid ?
or
Write down the relation among the linear , surface and volume expansion coefficients of a solid ?
2.6 What type of spherical mirror is used in the head light of cars ?
2.7 Where will the image be formed when an object is placed at the focus of a convex lens ?
2.8 Define the resistance of a conductor according to Ohm's law .
2.9 What is the SI unit of resistivity ?
2.10 From which part of an atom the radioactive rays emanate ?
or
Name a positively charged radioactive particle .
2.11 Match the right column with the left :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.12 Which type of electricity is used in electrolysis ?
2.13 What is used as electrolyte in the electroplating of gold over silver ?
2.14 Write the formula of the precipitate when an aqueous solution of ammonia is added to the aqueous solution of ferric chloride ?
or
What is liquor ammonia ?
2.15 What change of colour is observer when a few drops of aqueous solution of sodium nitroprusside is added to an aqueous solution of hydrogen sulphide made alkaline with NaOH solution ?
2.16 Write whether the following statement is true or false :
aqueous solution of glucose can conduct electricity .
2.17 write UPAC name of the following compound .
CH₃CH₂CHO
2.18 Which gas is liberated when Na HCO₃ is added to acetic acid ?
or
Write down the formula of the organic compound produced in the first step in the substitution reaction of methane with chlorine .
Group - C
3. Answer the following questions ( Alternative to be noted ) : 2x 9 = 18
3.1 Write the two harmful effects of depletion of ozone layer on human health and environment .
3.2 The volume of a gas at 300 K temperature and 760mmHg of pressure is 300 cm³ . What is the volume of the gas at STP ?
Or
Find out the volume of 2.2 g of CO₂ at temperature 300K and 570mmHg 0f pressure ( C = 12 , O = 16 , R = 0.082 Latmos .K⁻¹ mol⁻¹ )
3.3 Write the laws of refraction of light .
3.4 How does resistance depend of length and cross section of a conductor / ?
Or
Write down Fleming's left hand rule .
3.5 Draw Lewis -dot diagram of carbon dioxide .
Or
Show with an example that ionic can be formed even when its ions have no filled octet .
3.6 Compare two properties of ionic and co-valent compounds
3.7 Write the condition and balanced chemical equation of the reaction for the preparation of ammonia gas in the laboratory.
Or
Show that H₂S is a reducing agent with the help of a chemical reaction .
3.8 Write with example the difference between mineral and ore .
3.9 Write the names of monomers of PVC an teflon .
Or
Write constitutional formulas of two organic compounds having the same molecular formula C₂H₆O
Group -D
4. Answer the following questions ( Alternative are to be noted ): 3x 12 =36
4.1 Establish the combined form of Charles' law and Boyle's law.
4.2 How many gram of ferrous sulphide will be required to prepare 1.7 gram of hydrogen sulphide gas by the reaction of ferrus sulphide and dilute sulfuric acid ? ( Fe =56 , S = 32 , H = 1 )
Or
Metallic zinc and carbon monoxide are produced when zinc oxide is heated with carbon . How many gram of carbon will be required to produce 31.785 g of zinc and 14.000 g of carbon monoxide from 40.685 g of zinc oxide ? How many mole of carbon monoxide is produced in the reaction ? (C = 12 , O =16 )
4.3 What is meant by coefficient of thermal conductivity of a substance ? Establish the relation between CGS and SI units of it .
4.4 What is meant by focus of a lens ?
4.5 Prove that δ = i₁ + i₂ - A in case of refraction of light through a prism. (The symbols has usual meaning )
Or
If the refractive index of a medium for red and violet light are μ₁ & μ₂ respectively prove that μ₁ < μ₂
4.6 State Joule's law of heating of current .
4.7 When an electric lamp is connected to 220V mains 1 ampere of current flows . What will be the current when the lamp is connected to 110V mains ?
Or
Find the ration of the resistances of two lamps of 220V- 60W and 110V- 60W .
4.8 What is radioactivity ?
Write down two uses of radioactivity .
Or
When a radioactive atom emits an α particle how are atomic number and mass number changed in the daughter atom ?
By the emission of which radioactive ray from a radioactive atom , the atomic number remains unchanged ?
4.9 What is meant by ionization energy of an element ?
Arrange Li , Na and K in the increasing order of their ionization energy .
4.10 Mention the following points in case of electrorefining of copper .
i) electrodes used and the electrolyte
ii) the chemical reaction occurring at the electrodes .
4.11 In the laboratory preparation of nitrogen , why instead of heating directly a concentrated aqueous solution of ammonium nitrite , a mixed aqueous concentrated solution of ammonium chloride and sodium nitrite mixed in equimolar proportion is heated ? Answer with balanced chemical equations .
Or
Write with condition and balanced chemical equation how urea is industrially prepared .
4.12 C₂H₆ is called a saturated hydrocarbon , but why is C₂H₄ called an unsaturated hydrocarbon ?
Or
How will you convert HC≡CH → Br₂CHCHBr₂ ?
Mention one use of CNG
Answer keys :
Q 1 . To know answer copy the following link and paste it on your browser and take part in the quiz online .
Q2 . 1) Troposphere or Methanogens 2) decrease 3) -273 ºC 4) pressure will increase 5) K⁻¹ Or α =β/2 =४/3 6) concave mirror 7) at infinite distance 8) R =V/I , ratio of potential difference and current is resistance . 9) Ohm.metre 10) nucleus 11) 1. → iii) F 2. → iv) Mg 3. →i) Zn 4. → ii) Sn 12) direct current (D.C) 13) K[Au(CN)₂] 14) FeCl₃ +3 NH₄(OH) → Fe(OH)₃↓ + 3NH₄Cl Or aqueous solution of ammonia ( NH₄OH ) 15) purple colour 16) False 17) 1-propanal 18) CO₂ or CH₃Cl
Q3 .
3.1) Two harmful effects of depletion of ozone layer a) Ultra violet rays directly reach earth surface and causes skin cancer b) UV radiation hinders the growth of plants .
3.2) Here pressure is constant . Initial temperature T₁ = 300K ,initial volume V₁ = 300 cm³ final temperature T₂ = 0ºC = 273K , final volume V₂ = ? . From Charle' law V₁/T₁ = V₂ /T₂
⇒ 300/300 = V₂/273 Or , V₂ = 273 cm³
Or Molecular weight of CO₂ = 12 + 2x16 = 44 , 2.2 g of CO₂ = 2.2/44 = 0.05 mole . From PV =n RT we get 570/760 V = 0.05 x 0.082x 300 ⇒ V = 760 x 0.05 x 0.082 x 300/570 = 1.64 L
3.3) Laws of refraction : a) The incidence ray , the refracted ray and normal at the incident point all lie in the same plane .
₁μ₂ = sin i/sin r
3.4) Resistance is proportional to the length of conductor and inversely proportional to the cross-sectional area of conductor .
If we arrange our thumb , forefinger and middle finger of the left hand perpendicular to each other , then the thumb points towards the direction of the force experienced by the conductor , the forefinger points towards the direction of the magnetic field and the middle finger points towards the direction of the electric current .
3.6) Comparison between ionic and covalent compound :
|
|
|
|
|
|
|
|
|
|
|
|
3.7) Balanced equation for ammonia preparation in laboratory :
heat
2NH₄Cl + Ca(OH)₂ = CaCl₂ + 2H₂O +2NH₃(g)
Or
2FeCl₃ +H₂S →2 FeCl₂ + 2HCl + S
Here H₂S reduces FeCl₃ to FeCl₂ , Hence it acts as reducing agent .
3.8)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|